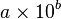- how many dollars is € 35.00 ? (1€ = $ 14)
€ 35 x $ 14/ € 1 = $ 49 - what is 100 km/h in miles/hour ? (1.6 km = 1 mi)
100 km/h x 1 mi/1.6km = 62.5 mi/h
- just like converting between currencies in chemistry it is usually necessary to convert between units
- this process is called dimensional analysis
STEPS:
- there are only 4 steps
- Identify what units you want to end up with
- Find the conversion factors
- Place units in the appropriate places
- Cancel units
EXAMPLES:
- How many miles are equal to 120 km ?
120 km x 1 mi/1.6 km = 75 mi
- How many seconds are in 1.4 hours ?
--> 1.4 h x 60 min/1 h x 60 s /1 min = 5040 s
--> 1.4 h x 3600 s/1 h = 5040 s
- What is 50 km/h in m/s ?
50 km/h x 1 h/3600 s x 1000 m/1 km = 13.88 m/s = 14 m/s
- Convert 150 kJ/h into J/s :
150 kJ/h x 1 h/3600 s x 1000 J/1 kJ = 41.7 J/s
- Convert 230 mg/s into g/min :
230 mg/s x 60 s/1 min x 1 g/1000 mg = 13.8 g/min
-Eva