A. There are 7 important periodic trends
1. Reactivity
2. Ion Charge
3. Melting Point
4. Atomic Radius
5. Ionization Energy
6. Electronegativity
7. Density*
II. REACTIVITY
A. Metals and non-metals show different trends
1. The most reactive elements are located on the outside of the periodic table
2. The elements on the inner part of the table are the least reactive
3. Note that noble gases are not reactive
III. Ion Charge
A. Elements ion charges depend on their group (column)

IV. Melting Point
A. Elements in the center of the table have to highest melting point
B. Elements closest to the outer edges have the lowest melting point
C. Noble gases have the lowest melting point
V. Atomic Radius
A. The atomic radius gets smaller and smaller as you move up and to the right
C. Francium has the largest atomic radius
VI. Ionization Energy
A. Energy required to move an electron from an atom
B.Ionization energy increases going from the right and going up
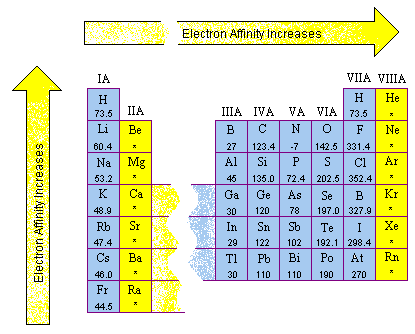
C. All noble gases have a high ionization energy
D. Helium has the highest ionization energy/ Francium has the lowest ionization energy
VII. Electronegativity
A. Electronegativity: how much atoms want to gain electrons
B. It increases going up and to the right

-Nicole P

No comments:
Post a Comment